We are solving the puzzle of functional protein fibrils for advancing applications in technology and medicine
The specific structural hallmarks that distinguish pathological from functional amyloids have long been unclear due to the relative lack of structural information available for the latter. Our lab determined the first crystal structure of a bacterial amyloid fibril. This structure, of the phenol soluble modulin α3 (PSMα3) peptide secreted by the pathogenic Staphylococcus aureus revealed a novel amyloid architecture of cytotoxic cross-α fibrils (Tayeb-Fligelman et. al., Science 2017). This presented a paradigm shift in amyloid research, as amyloids had been defined as cross-β fibrils. Moreover, we showed that fibrillation facilitates toxicity of this peptide towards human cells (Tayeb-Fligelman et al.,Structure 3;28(3):301-313; 2020), suggesting that amyloid formation plays a role in the pathogenicity of methicillin-resistant S. aureus (MRSA).
We recently revealed another cross-α structure formed by a eukaryotic antibacterial peptide (Salinas, Tayeb-Fligelman et. al., PNAS January 19, 2021 118 (3) e2014442118 ). The fields of antibacterial activity and amyloid pathology have indeed recently converged, raising hypotheses about proteins associated with neurodegenerative diseases fighting bacterial infections threatening the brain.
Interestingly, we found a truncated PSMα3 showing antibacterial activity, which also revealed atypical β-rich fibril architectures (Salinas et. al., Nature Communications 2018). This provided insight into new mechanisms of action of some antimicrobial peptides. Other members of the PSM family, PSMα1 and PSMα4, involved in biofilm structuring, formed cross-β amyloid fibrils similar to pathological amyloids, demonstrating that this architecture is conserved from bacteria to human (Salinas et. al., Nature Communications 2018). In microbes, these structures confer high stability to the biofilm matrix, leading to resistant infections. Overall, our investigations of the PSM family in S. aureus indicates structural plasticity of their fibrils being at the basis of their functional diversity (Landau, Nature Chemical Biology 2018).
In addition, by showing structure similarity between human pathological amyloids and a biofilm-associated amyloid from enterobacteria, we offered repurposing of anti-Alzheimer’s compounds as anti-biofilm agents (Perov et al., PLoS Pathogens 2019). These interesting, yet frightening findings, suggested that microbial amyloids could induce amyloid aggregation diseases via cross-seeding. This is reminiscent of transmissible prion diseases such as Creutzfeldt – Jakob disease and bovine spongiform encephalopathy (known as the Mad Cow disease).
The studies of probiotic and pathogenic microbial amyloid systems are crucial for understanding the basis of their diverse roles in infections, and pave the way for the design of novel antivirulence drugs that will address bacterial resistance and perhaps neurodegeneration and systemic amyloid diseases.
Our set of tools: X-ray microcrystallography, electron microscopy, light microscopy, biophysics, biochemistry, bioinformatics, microbiology and cell biology, and computational biology.
Highlights
The amphibian antimicrobial peptide uperin 3.5 is a cross-α/cross-β chameleon functional amyloid
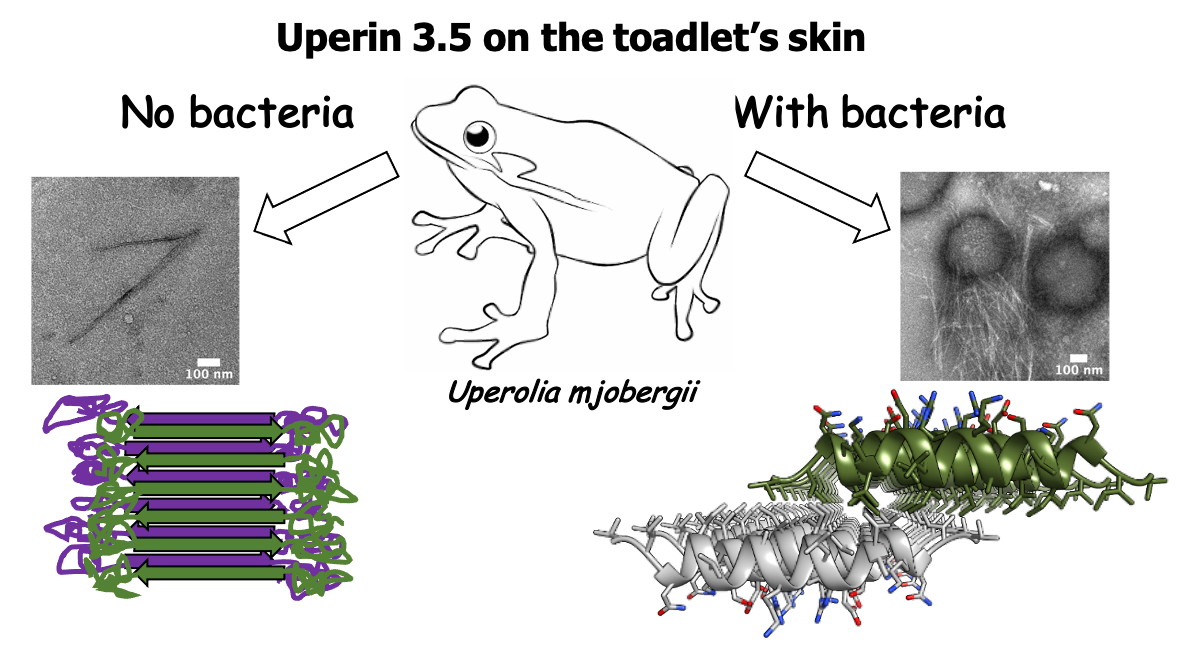
Nir Salinas*, Einav Tayeb-Fligelman*, Massimo D. Sammito, Daniel Bloch, Raz Jelinek, Dror Noy, Isabel Usón, Meytal Landau. PNAS 118 (3) e2014442118; 2021 DOI: 10.1073/pnas.2014442118. *Authors contributed equally.
The Human LL-37(17-29) Antimicrobial Peptide Reveals a Functional Supramolecular Structure
Image Credit: Sharon Amlani
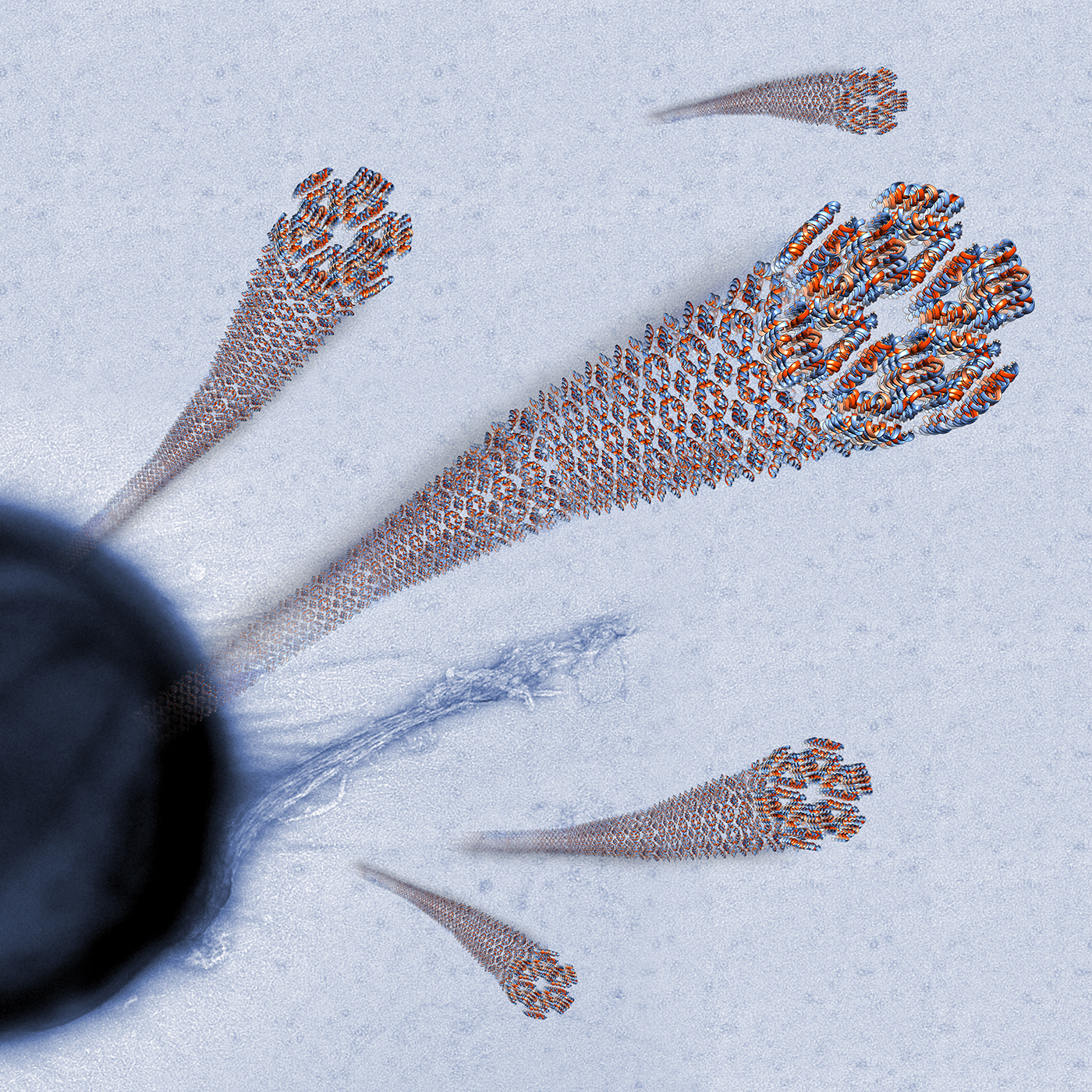
Engelberg and Landau. Nat Commun 11, 3894 (2020)
Structural similarity between amyloidogenic segments from enteric bacteria curli and human pathological amyloids open ways to new anti-biofilm agents
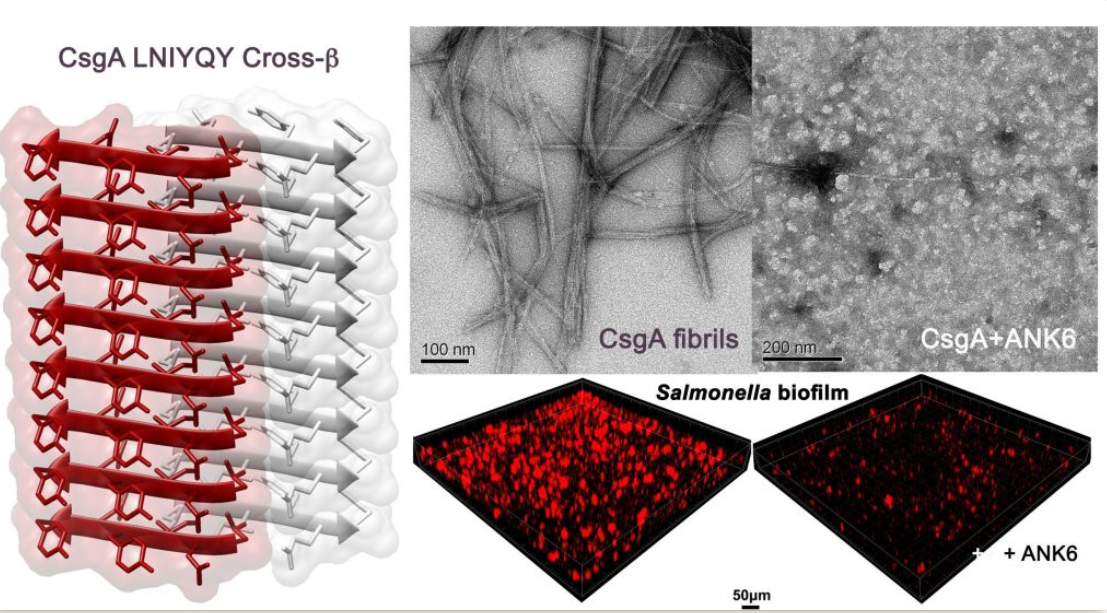
Perov et. al. PLoS Pathog 15(8): e1007978; 2019
Phenol Soluble Modulins (PSMs) secreted by the pathogenic S. aureus demonstrate a vast structural diversity of amyloid like structures encoding their functional diversity
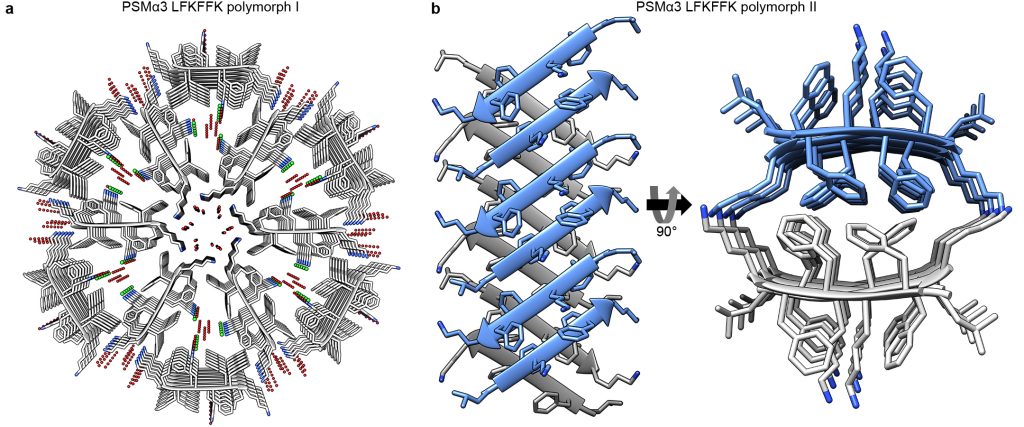 Salinas et. al. Nature Communications 9(1):3512; 2018
Salinas et. al. Nature Communications 9(1):3512; 2018
Cross-α amyloid-like fibrils secreted by the golden bacterium S. aureus are toxic to human cells

